MYOPIAX® CLINICAL TRIAL
What is the MyopiaX® clinical trial?
The purpose of this trial is to investigate the clinical effects of MyopiaX®
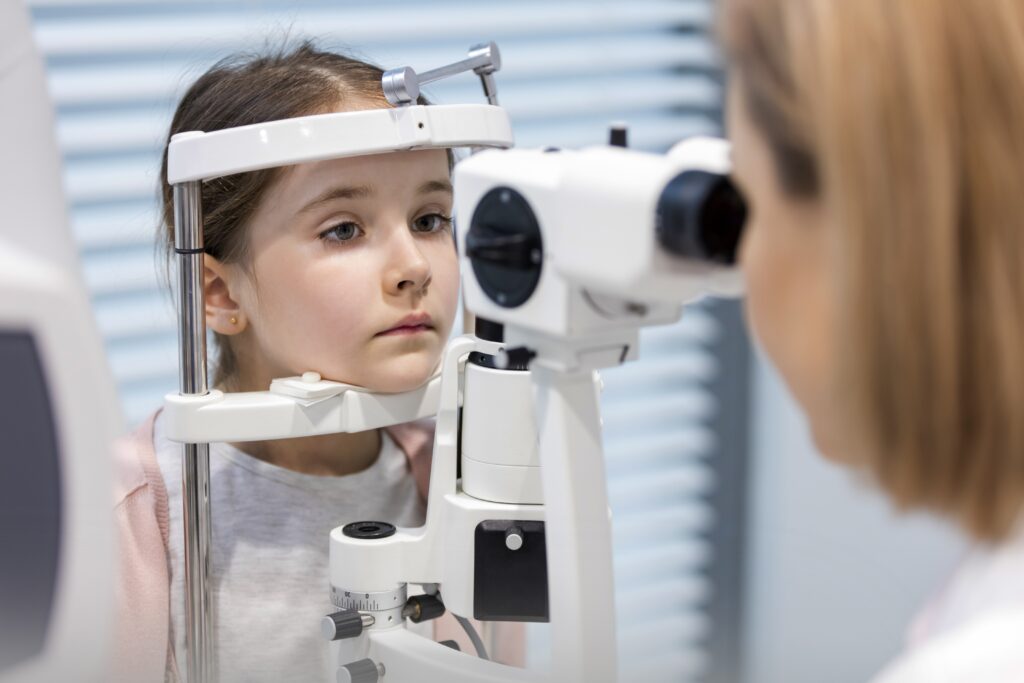
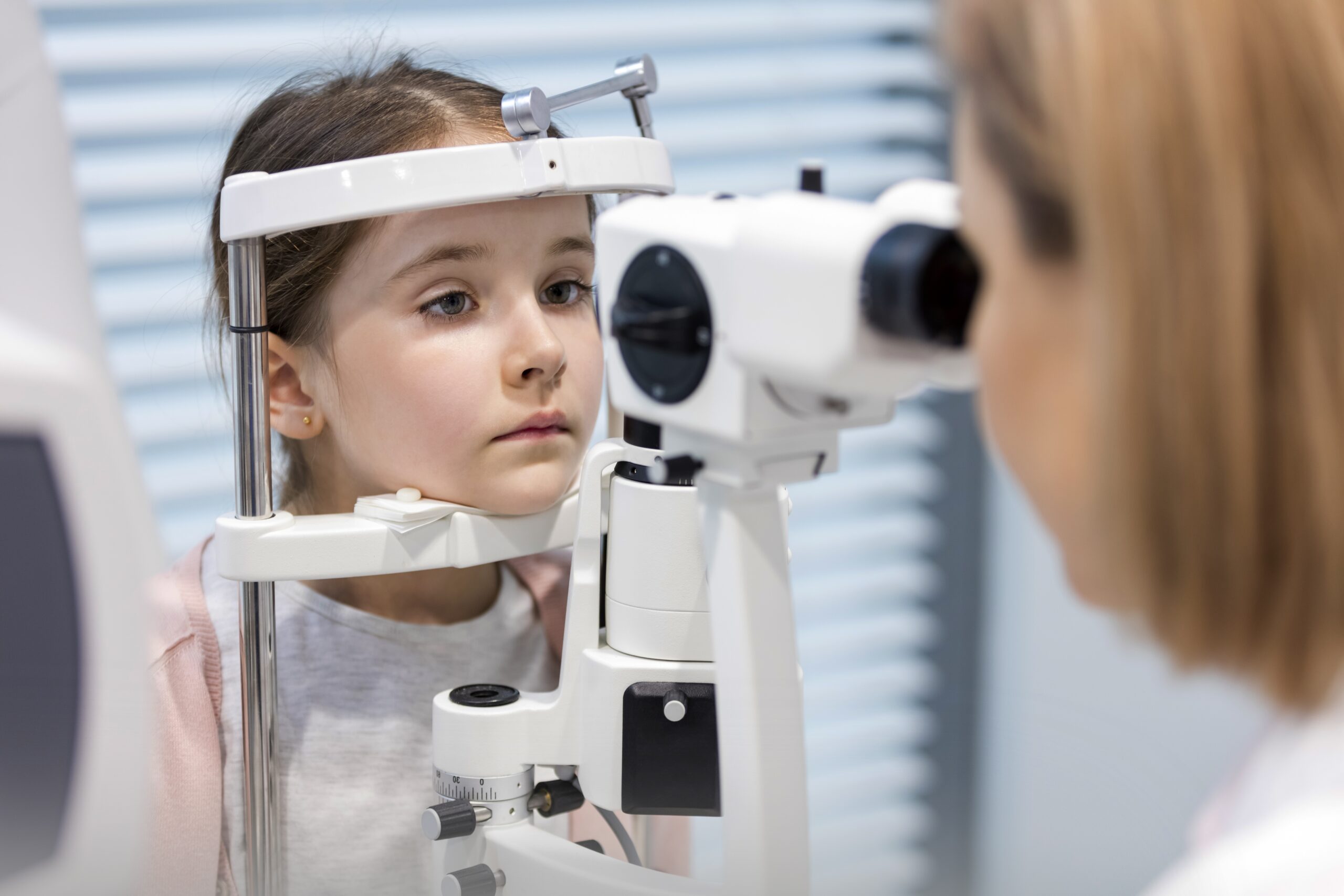
MyopiaX® is a smartphone application that aims to slow the progression (worsening) of myopia, or nearsightedness, in children and adolescents. We are conducting a clinical trial (NCT04967287) to study the safety and effect of treatment with MyopiaX® on myopia progression.
MyopiaX® delivers selective ocular light stimulation to the eye’s blind spot. Used together with a virtual reality headset and wireless controller, MyopiaX® aims to deliver a medical effect alongside an interactive and child-friendly digital experience.
The MyopiaX® clinical trial investigates the effects of MyopiaX® (Group 1) and myopia control spectacles (Group 2) on myopia progression.
The MyopiaX® clinical trial has been reviewed and approved by the regulatory authorities and ethics committees of the respective clinical institutions in each country to ensure the safety and well-being of participants throughout the trial.
The trial includes 10 recognized research and clinical centres in Germany, Spain, Portugal, the Netherlands, and the United Kingdom.
The MyopiaX® clinical trial achieved its 6-month milestone in April 2024 and is scheduled to conclude in September 2024.
WHO CAN PARTICIPATE?
The MyopiaX® trial includes myopic children between 6 and 12 with:
- A refractive error starting at -0.75 Diopters,
- No previous or current myopia treatment, or participation in other trials,
- No other eye conditions or illnesses affecting the eye,
- No medical or family history of photosensitive epilepsy.

How does it work?
- Children will be randomly assigned to use MyopiaX® (Group 1: 2 in 3, 67%) or myopia control spectacles (Group 2: 1 in 3, 33%).
- Group 1 will use MyopiaX® twice daily at home for 12 months. After six months, these children will also wear myopia control spectacles.
- Group 2 will not use MyopiaX®. Instead, they will wear myopia control spectacles for 12 months.
- There will be five clinic visits.

WHY PARTICIPATE?
- Participants may benefit from regular eye exams, which aim to help manage myopia and measure its progression.
- The myopia progression of participants may show signs of slowing due to the two treatments studied in this trial.
- Participants will be part of a group of peers from across Europe participating in the same study.
WHO CAN PARTICIPATE?
The MyopiaX® trial includes myopic children between 6 and 12 with:
- A refractive error starting at -0.75 Diopters,
- No previous or current myopia treatment, or participation in other trials,
- No other eye conditions or illnesses affecting the eye,
- No medical or family history of photosensitive epilepsy.

How does it work?
- Children will be randomly assigned to use MyopiaX® (Group 1: 2 in 3, 67%) or myopia control spectacles (Group 2: 1 in 3, 33%).
- Group 1 will use MyopiaX® twice daily at home for 12 months. After six months, these children will also wear myopia control spectacles.
- Group 2 will not use MyopiaX®. Instead, they will wear myopia control spectacles for 12 months.
- There will be five clinic visits.

WHY PARTICIPATE?
- Participants may benefit from regular eye exams, which aim to help manage myopia and measure its progression.
- The myopia progression of participants may show signs of slowing due to the two treatments studied in this trial.
- Participants will be part of a group of peers from across Europe participating in the same study.

Interested in learning more?
For more information, contact us at clinicaltrials@dopavision.com or get in touch with a research specialist from one of the clinics below. Researchers and clinicians are also welcome to use this e-mail address to reach out to us with questions or for more information.
Find a trial site
Augsburg
Südblick Augenzentrum, Makula & Dry Eye Center
myopia@suedblick.de
0821-41903010
Düsseldorf
MVZ Makula-Netzhaut-Zentrum Breyer Kaymak Klabe
Dr. Hakan Kaymak
netzhaut@augenchirurgie.clinic
+49 211 2730410
Köln
Praxis BeyondEye
praxis@beyondeye.de
Mainz
Universitätsmedizin der Johannes Gutenberg-Universität Mainz
Augenklinik und Poliklinik
Klinisches Studienzentrum
klinisches-studienzentrum-augenklinik@unimedizin-mainz.de
+49 613 1178356
Tübingen
University Eye Hospital Tübingen
Rotterdam
Erasmus University Medical Center
Trialbureau Oogheelkunde Erasmus MC
onderzoek.oogheelkunde@erasmusmc.nl
+31 10 7039740
Barcelona
Hospital Sant Joan de Déu
+34 933 53 21 00
Madrid
University Complutense of Madrid
Ocupharm Research Group
investigacionesocupharm@gmail.com
+34 611 63 84 34
United Kingdom

The MyopiaX® clinical trial is including…
- Children between the ages of 6 and 14 years with progressive myopia starting at -0.75 Diopters
- Six visits to the clinic over a 24-month period
- At-home treatment two times every day
- Five scheduled phone call evaluations between the visits, from the research specialist at the clinic
- A 12-month follow-up period after the end of the 24 months treatment, with two additional visits to the clinic
- Six research clinics located in Ireland, Germany, Portugal, Spain, and the Netherlands.
The study has been reviewed and approved to be conducted by the regulatory authorities and ethics committees of the respective clinical institutions in each country to ensure participants’ safety and well-being throughout the trial.